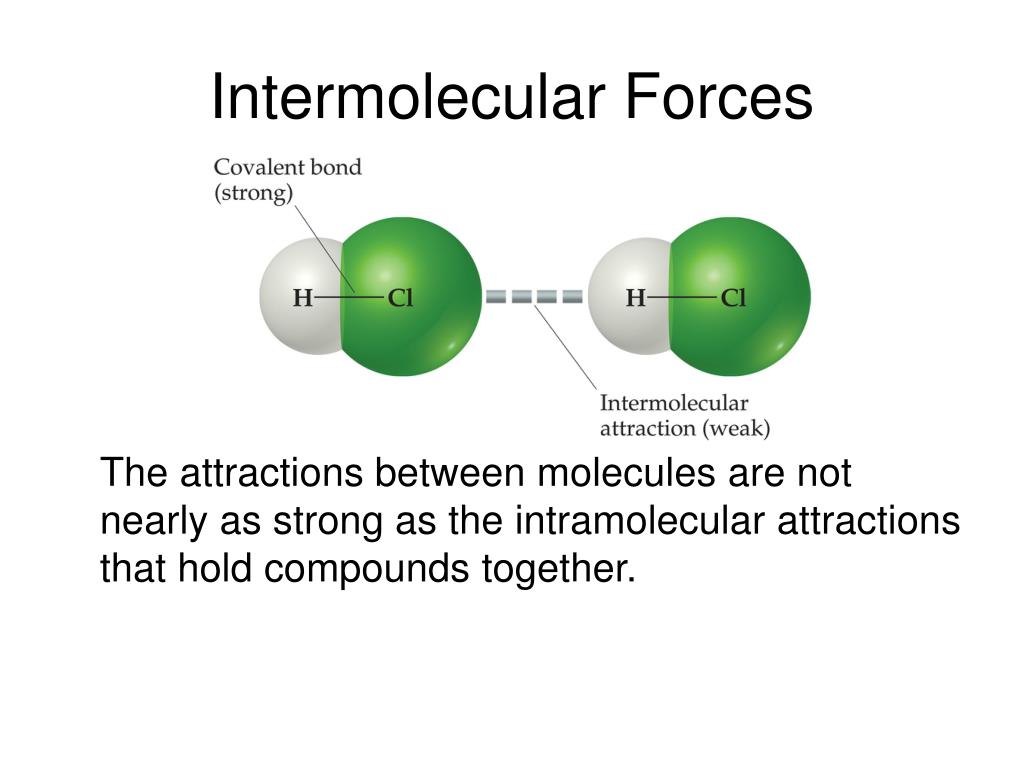
What Are The Intermolecular Forces In Hcl . All of the attractive forces between neutral atoms. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times.
from www.slideserve.com
inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. All of the attractive forces between neutral atoms.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
What Are The Intermolecular Forces In Hcl All of the attractive forces between neutral atoms. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. there are three types of intermolecular forces: All of the attractive forces between neutral atoms. inter molecular forces hold multiple molecules together and determine many of a substance’s properties. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between.
From www.clutchprep.com
Intermolecular Forces Chemistry Video Clutch Prep What Are The Intermolecular Forces In Hcl there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces are forces between molecules, in the same way that an intercontinental. What Are The Intermolecular Forces In Hcl.
From www.siyavula.com
4.1 Intermolecular and interatomic forces Intermolecular forces What Are The Intermolecular Forces In Hcl inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. All of the attractive forces between neutral atoms. inter molecular forces hold multiple molecules together and determine many of a substance’s properties. there are three types of intermolecular forces: however, to break the covalent bonds between the hydrogen. What Are The Intermolecular Forces In Hcl.
From garymeowhogan.blogspot.com
Hcl Intermolecular Forces What Are The Intermolecular Forces In Hcl inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. All of the attractive. What Are The Intermolecular Forces In Hcl.
From jsmithmoore.com
Intermolecular forces What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many. What Are The Intermolecular Forces In Hcl.
From learningschoole1ja3t2u3k.z22.web.core.windows.net
Intermolecular Forces Explained Simply What Are The Intermolecular Forces In Hcl All of the attractive forces between neutral atoms. there are three types of intermolecular forces: inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular. What Are The Intermolecular Forces In Hcl.
From courses.lumenlearning.com
Intermolecular Forces Chemistry What Are The Intermolecular Forces In Hcl there are three types of intermolecular forces: All of the attractive forces between neutral atoms. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular. What Are The Intermolecular Forces In Hcl.
From studiousguy.com
Intermolecular Force Types and Examples StudiousGuy What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many. What Are The Intermolecular Forces In Hcl.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. All of the attractive forces between neutral atoms. there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many of a substance’s properties. inter molecular forces are forces between. What Are The Intermolecular Forces In Hcl.
From studylib.net
Intermolecular Forces What Are The Intermolecular Forces In Hcl inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. All of the attractive forces between neutral atoms. there are three types of intermolecular forces: inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen. What Are The Intermolecular Forces In Hcl.
From www.chemistrylearner.com
Intermolecular Forces Definition, Types, and Examples What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces hold multiple molecules together and determine many of a substance’s properties. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three. What Are The Intermolecular Forces In Hcl.
From www.youtube.com
CHEMISTRY 101 Identify intermolecular forces and discover their What Are The Intermolecular Forces In Hcl inter molecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between neutral atoms. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three types of intermolecular forces: however, to break the covalent bonds between the hydrogen. What Are The Intermolecular Forces In Hcl.
From techiescientist.com
CH3OH (Methanol) Intermolecular Forces Techiescientist What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. All of the attractive forces between neutral atoms. inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. inter molecular forces hold multiple molecules together and determine many. What Are The Intermolecular Forces In Hcl.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Are The Intermolecular Forces In Hcl inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. there are three types of intermolecular forces: however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces hold multiple molecules together and determine many. What Are The Intermolecular Forces In Hcl.
From thechemistrynotes.com
Intermolecular Forces Definition and Types What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. there are three types of intermolecular forces: inter molecular forces are forces between molecules, in the same way that an intercontinental missile can fly between. All of the attractive forces between neutral atoms. inter molecular. What Are The Intermolecular Forces In Hcl.
From santostinstokes.blogspot.com
Hcl Intermolecular Forces SantostinStokes What Are The Intermolecular Forces In Hcl inter molecular forces hold multiple molecules together and determine many of a substance’s properties. All of the attractive forces between neutral atoms. there are three types of intermolecular forces: however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces are forces between. What Are The Intermolecular Forces In Hcl.
From www.numerade.com
SOLVED Part A What is the predominant intermolecular force in the What Are The Intermolecular Forces In Hcl inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. All of the attractive forces between neutral atoms. inter molecular forces are forces between molecules, in the same way that an intercontinental. What Are The Intermolecular Forces In Hcl.
From techiescientist.com
HCl Intermolecular Forces — Type, Strong or Weak? Techiescientist What Are The Intermolecular Forces In Hcl however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. there are three types of intermolecular forces: All of the attractive forces between neutral atoms. inter molecular forces hold multiple molecules together and determine many of a substance’s properties. inter molecular forces are forces between. What Are The Intermolecular Forces In Hcl.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Are The Intermolecular Forces In Hcl there are three types of intermolecular forces: All of the attractive forces between neutral atoms. inter molecular forces hold multiple molecules together and determine many of a substance’s properties. however, to break the covalent bonds between the hydrogen and chlorine atoms in one mole of hcl requires about 25 times. inter molecular forces are forces between. What Are The Intermolecular Forces In Hcl.